Securing biopsies for further research
To secure high-quality biopsies for research, several conditions have to be taken into account. It’s especially important to monitor the temperature of the samples, sufficiently trace the samples, and to get consent for further analysis. Automating and standardizing biopsy collection, transportation and storage improves efficiency and reduces errors.
Monitoring the temperature of samples
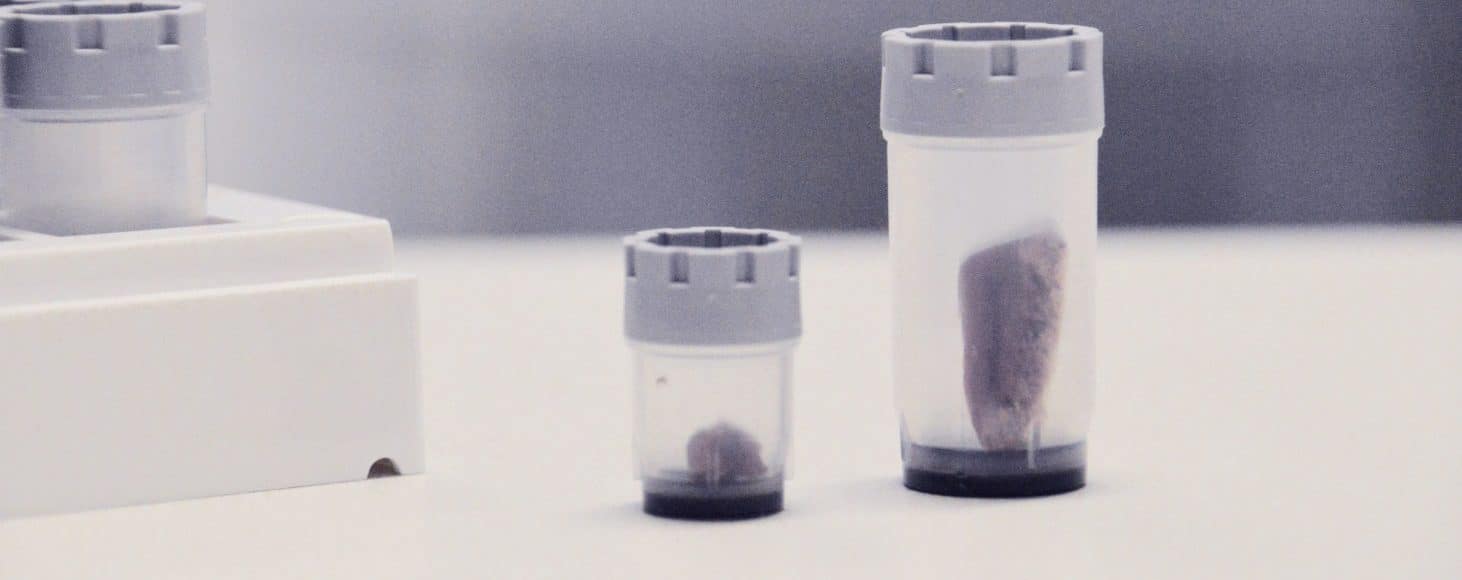
Varying temperatures can influence the quality of samples. Consequently, hospitals and biobanks control the temperatures to which a biopsy is exposed. For example, IBDW (Interdisciplinary Bank of Biomaterials and Data Wuerzburg) regulates the temperature of each tissue sample that it processes [1]. At IBDW, biopsies move from the patient to the storage facility at room temperature, samples are stored in -80°C freezers and transported at -80°C on dry ice [1]. IBDW uses Micronic Tissue Tubes for the transportation and storage of biopsies at these temperatures [1]. Micronic tubes are specifically designed to store and transport frozen tissue samples. Due to thick tube walls, Micronic tubes have a sturdy design and excellent properties for ultra-low temperature storage.
Tracing biopsies for research
When taken from a patient, biopsies should be labeled to ensure that the sample can be traced from the operating table to the storage freezer. Larger hospitals process thousands of samples each month. To prevent mixing up samples, each sample should have a unique identification code. Scanning, processing and storing these codes should happen with standardized procedures.
A Laboratory Information System (LIMS) can track samples in automated and/or manual freezers. Scanning the unique codes of sample storage tube enables researchers to add new samples to the database. Micronic laser-etches 2D Data-Matrix codes on their tubes so that they can never wear or fall off. This guarantees the traceability of samples by eliminating the risk that a code is separated from a sample.
Consent for further analysis
Maintaining sample integrity and securely tracing samples is important for further research; however, to use biopsies for research, it’s also essential to have the consent of a patient. The study of Geiger, Both, Kircher, Neumann, Rosenwald, and Jahns (2018) explains how broad consent is useful to collect samples for various research applications [1]. By securing as many biopsies for research as possible with the right procedures, scientists are able to do a higher frequency of better quality research.
[1] Geiger, J, Both, S, Kircher, S, Neumann, M, Rosenwald, A and Jahns, R. 2018. Hospital-integrated Biobanking as a Service – The Interdisciplinary Bank of Biomaterials and Data Wuerzburg (ibdw). Open Journal of Bioresources 5: 6, DOI: https://doi.org/10.5334/ojb.38